|
Return
Copied
from Ref listed at end of page with
some recent photos added to the drawings.
CONDUCTION OF ELECTRICITY THROUGH
GASES
Advance Laboratory Practice In Electricity and
Magnetism By Earle Melvin Terry McGraw Hill Book Co. 1st
edition 1922
149.
Electrons.—When a high tension discharge passes
between electrodes
sealed into a partially evacuated vessel, the gas becomes luminous showing
a series of highly colored glows which are often very beautiful. If
the pressure is sufficiently reduced, a series of streams appears,
proceeding in straight lines from the cathode. These streams are
known as "cathode rays," and are found to be independent of the position
of the anode, and often penetrate regions occupied by other glows in the
tube. The researches of modern physics have shown that
these rays are streams of discreet particles of negative electricity,
called "electrons." Their properties do not depend upon the
material of the electrodes nor the nature or presence of the gas
through which the discharge takes place. They may be produced from
all chemical substances, and consequently must play an important part in
the structure of matter. The velocities with which they move through
the tube vary from one-thirtieth to one-third that of light. The
ratio of the charge of an electron to its mass is constant and is equal to
1.77 X 10^7
electromagnetic units per gram. The charge of an electron is 1.5 X
10^-20
electromagnetic units and The
mass is about 1/1800
that
of
the hydrogen
atom. The radius of an electron is estimated, at 1.9 X 10^-13
cms.,
which is about 1/50 000 that of the atom.
For
many
years the mass has been regarded as purely electromagnetic in
character; that is, while exhibiting inertia, it shows no gravitational
attraction in the sense possessed by ordinary matter. Recently,
however, certain experimental and theoretical evidence has been produced
which makes it appear likely that this cannot be entirely the case.
Many attempts have been
made to
discover evidence of quantities of electricity smaller or larger
than the electron, but none smaller have ever been found. In fact,
when quantities comparable to the electron have been isolated, they have
always proved to be exact integral multiples of it. The evidence
points to the conclusion that electricity is atomic in structure and
that the smallest possible element is the electron, which thus constitutes
our natural unit of electricity. Electric currents
through conductors, as we know them in every day practice, are
simply streams of electrons through or between the atoms and
molecules making up the conducting body.
150. Conductivity of Gases.—A gas in its normal state is
one of the best insulators known. This may be shown by mounting a
gold leaf electroscope inside an enclosed space, and allowing only a small
rod carrying a polished knob, for the purpose of charging, to project out.
If the support carrying the electro- scope is well insulated from the
container, the electroscope will remain charged for a long time, showing
that the air or what- ever gas surrounds the electroscope is a poor
conductor of electricity. If, however, X-rays are
allowed to shine through the enclosure, or if a small quantity of some
radio-active substance such as thorium or radium is placed inside it, or
again if the products of combustion of a flame are drawn through it, it is
then found that the gold leaves collapse quite rapidly, indicating that
the gas has lost its insulating
properties. That the leakage has taken place through the air
and not across the insulating support may be shown by using a second
chamber connected with the electrometer enclosure by a glass tube, and
introducing the X-rays, the radio active substance or other agent into
this, and then drawing the air thus acted upon into the first chamber.
The same effects are observed. However, if glass wool is introduced
in the connecting tube, or if the air is passed between two insulated
plates connected to a battery before entering the electrometer chamber, it
is found that its insulating properties are restored.
Experiments of this sort as well as many others of an entirely
different nature have shown that the conduction of electricity
through gases is due to carriers of electricity, and that the carriers are
of two distinct types, positive and negative; the former are similar to
the carriers of electricity through solutions and are called positive
ions, while the latter are either negative ions or electrons.
151. Structure of the Atom—To explain the phenomena of the
conductivity of gases, it is necessary first to make a brief statement
concerning the structure of the atom. While our knowledge is far
from complete, it is well established that the atom consists of a nucleus
of positive electricity, about which revolve in closed orbits, electrons,
in much the same way that the planets revolve about the sun, and that the
relative dimensions
of electrons, nucleus and orbits are about the same as in the solar
system. The number of electrons present in a given atom has been
estimated in various ways, and while the results are not entirely in
agreement, it is probable that it is the same as the atomic number, that
is, its number in the list of elements arranged in order of ascending
atomic weights. The atomic number, except for the case of hydrogen,
is approximately half its
atomic weight. Since the atom as a whole is neutral, it is necessary
that the positive nucleus should have a charge equal to
ne, where
e
is the charge of the electron and n the number of electrons.
The shape of the orbits, the law of force between nucleus and electron,
and even the conditions of stability are problems which have not yet been
solved, but are now being attacked from many angles.
When external agencies such as X-rays, ultra
violet light, radiations
from radio active materials, etc., act upon a gas, it is found that
the atomic structure is broken up. One or more electrons may be torn
away from the system leaving it with an excess of positive electricity.
We thus have present in the gas positive ions
and negative electrons. The gas is then said to be ionized, and
the means by which this condition is brought about is called the "ionizing
agent." If two electrodes are introduced, and a difference of
potential is maintained between them, the electrons move to the positive
electrode, and, entering it, pass on through the external metallic
circuit. The positive ions, on the other hand, move to the negative
electrode and receive electrons from it, thus becoming again neutral
molecules. Unless an ionizing
agent acts continuously, the current through the circuit will
persist only until the ions and electrons have been removed from
the gas.
152. The ionization
Current—Suppose now that an ionizing agent
is acting continuously upon a gas in an
ionization
chamber as an arrangement such as that just described is called.
At first it
might be supposed that if the agent acts long enough all of the
atoms would be ionized. This, however, is not the case; for, due to
their undirected heat motion, ions and electrons collide, and
recombine.
When the rate of recombination is equal to that of
ionization, a
steady state is reached where only a definite fraction, usually a
very small number, of the total number of molecules are in the ionized
state. If the difference of potential between the plates is varied,
and the current between them is measured and plotted as a function of
voltage, it is found that the current increases with the voltage almost
linearly at first, in accordance with Ohm's law; but for higher voltages,
the curve is concave downward and when a
certain voltage has been reached, no further increase in
current can be obtained, unless the voltage is raised to very large
values. The constancy of the current is due to the fact that all of
the ions and electrons produced are swept out by the field. This
current is spoken of as the "saturation current," from the similarity
between the shape of this curve and the magnetization curve for iron.
The voltage at which the horizontal part of the curve begins is called
the "saturation voltage." If the distance between the
electrodes is increased, it might, by analogy with metallic conductors, be
thought that the saturation current would be reduced because of the
increased path the ions and electrons must travel. It is found,
however, that the cur- rent is actually increased. This is because
there is a larger number of gas molecules subjected to the action of the
ionizing agent, and hence more carriers are produced. Again, it is
found that if the pressure of the gas is increased, the ionization
current is increased. Both of these facts show that the
saturation cur- rent through a gas is proportional to the mass of the gas
between the electrodes.
153. Ionization by Collision.—If the voltage
between the plates
of the ionization chamber is increased to sufficiently large values,
the saturation current does not remain constant indefinitely, for fields
may be reached
at which the current again begins to rise, slowly at first and then
very rapidly, finally resulting in a
disruptive spark accompanied by the passage of a current of
considerable magnitude. The field required for this
increased current depends upon the distance between electrodes, their
size and shape, and the nature and pressure of the gas. For air
at atmospheric pressure and spherical electrodes of moderate dimensions,
e.g., 1 cm. diameter, centimeter.
It is the order of 10,000 Volts per centimeter
It diminishes,
however, as the pressure is reduced, and is most conveniently
studied at pressures below 10 millimeters of mercury.
This increase in current is due
to the fact that ions are produced by collisions taking place
between neutral molecules
and ions as well as electrons already existing in the
gas. The mechanism of this process is somewhat obscure, but
it is clear that a definite amount of energy is required
to disrupt a neutral atom. The kinetic
energy of motion of the ions and electrons
depends upon how far they have moved under the accelerating field
before being stopped in the same way that
the energy of motion of a freely falling body depends upon the distance
through which it has fallen before being arrested. Thus, as the
pressure of the gas is reduced, the average length of free travel is
greater and the acquired energy available for ionizing purposes is
increased. The conductivity of a gas therefore
increases as the pressure is reduced. Since, however,
the conductivity depends upon carriers which come
originally from neutral molecules, the conductivity
can not increase indefinitely with decrease of pressure, for the
effect of the decreased available supply will eventually be felt.
An optimum pressure therefore exists at
which the increased range for acceleration is just balanced by the
decreased supply of molecules. For air, this pressure is of the
order of a few tenths of a millimeter of mercury. A further decrease
in the pressure results in a rapid increase in the resistance of the
gas. If a perfect vacuum could be obtained, the free space
between electrodes would be a perfect insulator. While this is, of
course, impossible, it is, nevertheless, easy with modern methods
of evacuation to obtain pressures so low that no appreciable discharge can
be detected with the highest fields available in
the laboratory. ________________________________________________
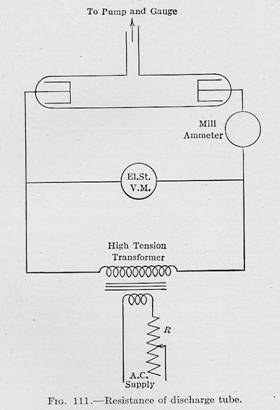
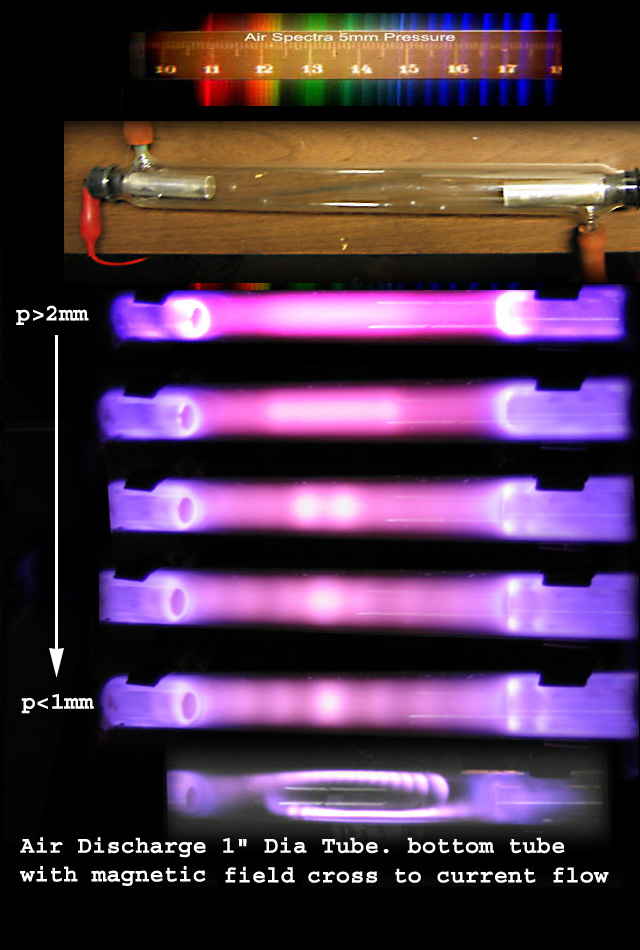
A circuit diagram below showing an experimental set up to observe the
visible electric and magnetic characteristics of gases brought to
high temperature by electric current passing through the gas a low
pressures.

-
Above is a drawing of a discharge tube running on
a steady direct current (d.c.)showing the curious bright and dark
band and its cathode dark spot..
-
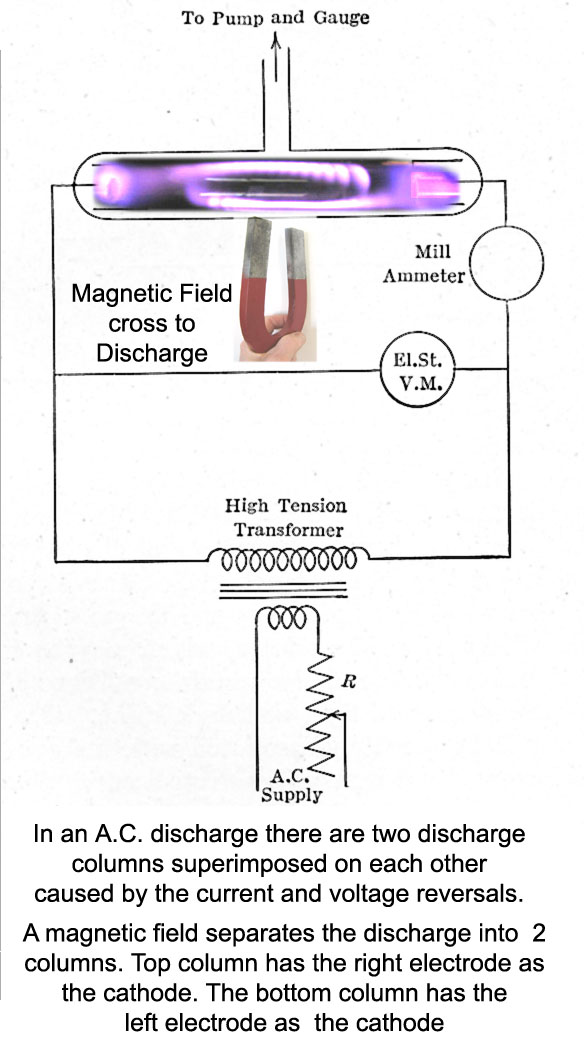
If the tube is run on 60 cycles per second alternating
current the polarities of the electrodes change from + to - 60 times a
second giving two discharge columns which over lap each other thus
blending. out to some extent the dark cathode feature. Since this
change goes back and forth 60 times a second the eye sees both at the same
time, i.e. they're blend together.
-
Using a magnet with its magnetic field perpendicular to
the arc current flow separates the two columns so that one can see
each arc column with its cathode dark space.
( Why does a magnetic field
do this?)
Below is the same diagram as above except a photograph was added showing a
discharge tube running on alternating current (a.c.) with a magnet
placed perpendicular to the current flow demonstrating the effect.

______________________________________________________
Again
155. Phenomena of the Discharge Tube Running on Direct Current—If
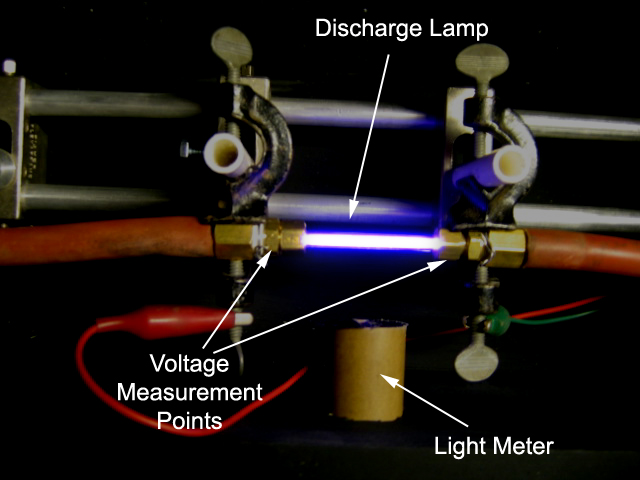
electrodes are mounted at the ends of a tube such as shown in Fig. 112,
containing air
at ordinary pressures and a sufficiently high voltage is
impressed between them, the phenomenon first observed is the ordinary
spark similar to that between the electrodes of a static machine.
If, however, air is gradually removed, the sparks become less violent, and
fine streamers of bluish color are observed. As the pressure is further
reduced, these streamers broaden out and fill the entire tube, and a pink
color appears at the anode. With further exhaustion, the pink color
extends some distance from the anode and dark spaces appear in the region
of the cathode. When the pressure has been reduced to
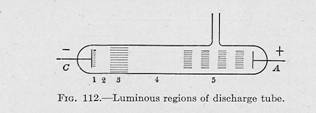
Direct Current above
Alternating Current Below
about half a millimeter of mercury, the discharge
assumes a very characteristic appearance. Closely
surrounding, but not quite touching the cathode, is a thin layer of
luminosity known as the cathode glow. Next to this is a
region, from which no light is observed, called the
Crooke's
dark space, and beyond this is a rather broad region of
luminosity known as the negative glow. Following this is another
non-luminous region, called the Faraday dark space.
Between this dark space and the positive electrode is a region called the
positive column, which may be seen as a continuous band of light or,
under certain conditions of current and voltage, as a series of light and
dark striae. The positive column seems to be definitely associated
with the anode, for if the tube is increased in length or bent into a
curve, the positive column increases or bends with it, while the other
parts of the discharge remain fixed and are thus shown to be associated
with the cathode. These luminous regions are indicated in Fig. 112.
If the pressure is still further reduced, the striae of the
positive column become fewer in number and wider in extent and finally .disappear.
The regions associated with the cathode also become larger and,
with the disappearance of the positive column, the dark spaces fill nearly
the entire tube. With sufficient exhaustion, the
Crooke's dark
space completely fills the tube, and the voltage required for a
passage of current becomes very high. At this stage, the walls of
the tube fluoresce
brilliantly with colors depending upon its chemical
composition, being bluish for soda, and bright green for German glass.
If the exhaustion is carried far enough, the tube becomes a non- conductor
of electricity.

156. Theory of the Discharge.—Since
no external ionizing agent is acting, it is obvious that the
discharge is maintained by ions produced by collision, and the varied
distributions of the luminous regions indicate that the electric fields
and the velocities of the carriers can not be uniform throughout the tube.
It has not yet been definitely determined whether luminescence arises from
ionization
of neutral molecules or whether it accompanies the recombination of
an ion and an electron to form a neutral molecule. At the present
time, the evidence seems to favor the latter hypothesis. Another
widely accepted view is that when a molecule has been shaken up by
collision with an ion or electron to such an extent that its electronic
orbits are badly distorted, but not disrupted, light emission accompanies
its return to the equilibrium state. On the latter theory, luminous
regions do not necessarily coincide with regions of ionization. Some
of the more important phenomena characterizing the several regions
enumerated above are the following. 1. Cathode
Glow.—The field strength in this region is large and often the greater
part of the entire potential difference occurs in this limited space.
The magnitude of the fall in potential depends upon the nature of the gas
and the material of the electrode, ranging from 470 volts for water vapor
to 170 volts for argon with platinum electrodes. If metals such as
magnesium, sodium, or potassium are used, much smaller values are obtained
because of the greater ease with which these substances emit
electrons. The large potential gradient here is caused by the
accumulation of positive ions in front of the cathode. Because of
the greater mobility of electrons, they rapidly move away from this
region thus leaving a preponderance of positive ions. The
ionization is caused by collision of the positive ions either with gas
molecules or the cathode itself. 2.
Crooke's
Dark Space.—It was pointed out above that a certain amount of energy
is required to produce ionization. The electrons from the cathode
glow must move through a certain difference of potential before they possess
the requisite kinetic energy for this purpose. The
Crooke's dark
space represents this distance for it is here that electrons,
liberated in the cathode glow, are acquiring the necessary energy of
motion to produce the ionization
of the negative glow. It is, in general, a rough measure of
the mean free path of the electrons. No
ionization
occurs in this region and the current is carried almost exclusively
by the electrons. 3. Negative Glow.—The luminosity of
this region is due to ionization by electrons from the Crooke's dark
space. The positive ions produced here move slowly out of the
negative glow into the Crooke's dark space and their presence reduces the
potential gradient to such an extent that electrons, originating in the
negative glow, do not gain sufficient speed to produce ionization;
and hence, after those entering from the Crooke's dark space have
been stopped by the ionization process, no further ionization occurs.
4. Faraday Dark Space.—The current in this region is
due largely to electrons which enter it from the negative glow. Because of
the accumulation of electrons in the negative glow, the potential gradient
through the Faraday dark space and even up to the anode is quite large.
The electrons are accordingly accelerated through this dark space and when
they have attained velocities
sufficient for ionization, the positive column commences.
5.
Positive Column.—The potential gradient is
practically constant throughout this region and ionization by collision
may take place all the way, resulting in a uniform column of
light. Ordinarily, however, there are local accumulations of
positive ions, which result in a decrease in the potential gradient with
a consequent reduction in the acceleration of the electrons.
There are then regions in which the velocities are too small to produce ionization
and the striae commonly observed, result. Under these
circumstances, the positive column is, to a certain extent, a repetition
of the phenomena of the Crooke's dark space, and the negative glow.
. References:
1
CBOWTHEB, Ions, Electrons and Ionizing
Radiations. McCMJNG,
Conduction of Electricity through Gases and Radioactivity.
MILLIKIN, The
Electron. THOMSON, Discharge of Electricity through
Gases.
TOWNSEND,
Electricity in Gases. GRAHAM,
Wied.
Ann.,
vol. 64, 1898, p. 49.
'
CROWTHER, Ions,
Electrons, afad
Ionizing Radiations, chap. VI.
TOWNSEND, Electricity in Gases, chap.
XI.
The particles of Modern physics by
J.D.Stranathan
1948 The Blakiston Philadelphia
chapter 3
The Electric Discharge pages 65
through 100
hello this is my first attempt at dictating
into an application I am quite surprised at how well it's doing .
Just likes science depends upon length mass and time this
application depends upon a very precise way of speaking.
|









